Alzheimer dementia
Diagnostics
Clinical criteria for Alzheimer’s dementia (McKhann et al, 2011)
1) Probable Alzheimer’s disease dementia (core clinical criteria)
- a. Insidious onset over months to years
- b. Clear-cut history of worsening cognition by report or observation
- c. Initial and most prominent cognitive deficits on history and examination are one of the following:
- i. Amnestic presentation: impairment in learning and recall
- ii. Non-amnestic presentation
- 1. Language presentation: word-finding difficulties, deficits in other domains should be present
- 2. Visuospatial presentation: spatial cognition-object agnosia, facial recognition, simultagnosia, and alexia, deficits in other domains should be present
- 3. Executive dysfunction: impaired reasoning, judgment and problem solving, deficits in other domains should be present
- d. There is no evidence of
- i. Stroke temporally related to the onset of cognitive symptoms or presence of extensive infarcts or severe white matter hyperintensity burden
- ii. Core features of DLB other than dementia itself
- iii. Prominent features of bvFTD
- iv. Prominent features of semantic or non-fluent / agrammatic PPA
- v. Other active neurological disease, medical comorbidity, or use of medications with effects on cognition
2) Probable AD dementia with documented decline: core clinical criteria + evidence of decline on subsequent evaluation based on informants, formal neuropsychological evaluation, or standardized mental status examinations
3) Probable AD dementia in a carrier of a causative AD genetic mutation: core clinical criteria + presence of APP, PSEN1, or PSEN2 mutations
4) Probable AD dementia with evidence of AD pathophysiological process meets core clinical criteria + biomarker data
- a. High probability: amyloid PET or CSF + positive CSF tau, FDG-PET, or structural MRI
- b. Intermediate probability:
- i. unavailable, conflicting, or indeterminate amyloid PET or CSF + positive CSF tau, FDG-PET, or structural MRI
- ii. positive amyloid PET or CSF + unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
- c. Uninformative: unavailable, conflicting, or indeterminate amyloid PET or CSF + unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
5) Possible AD:
- a. Atypical: meets core clinical criteria for AD but either has a sudden onset or demonstrates insufficient historical detail or objective cognitive documentation or progressive decline
- b. Etiologically mixed presentation meets criteria for AD but has evidence of
- i. Stroke
- ii. Features of DLB other than dementia
- iii. Evidence of another neurological disease or medical condition with effects on cognition
6) Possible AD dementia with evidence of AD pathophysiological process: atypical clinical presentation plus the following biomarker data:
- a. High probability: positive amyloid PET or CSF + positive CSF tau, FDG-PET, or structural MRI
- b. Intermediate probability
- c. Uninformative: unavailable, conflicting, or indeterminate amyloid PET or CSF + unavailable, conflicting, or indeterminate CSF tau, FDG-PET, or structural MRI
Variants
There are four common clinical presentations in Alzheimer's disease (Salardini 2019)
- Amnestic: typical problems with episodic memory (other domains may be affected, most commonly visuospatial cognition such as the inability to navigate), early changes in mesial temporal lobe,
- Visual variant (posterior cortical atrophy) Alzheimer dementia: visual perception is compromised in the absence of any ophthalmological problem, typically presents in mid-50's or early 60's, relative sparing of other domains not involved in vision, neuroimaging with occipito-temporal atrophy
- Logopenic variant primary progressive aphasia: impairment in phonemic lexicon, leading to impaired naming, hesitation of speech, and spelling changes; memory deficits and anxiety often coexist
- Executive variant or frontal AD: executive dysfunction and behavioral symptoms, shares overlap with bvFTD
Autosomal dominant Alzheimer disease: PSEN1, PSEN 2, and APP genes are known autosomal dominant genes in AD - see Genetics of Alzheimer disease subsection
Early onset Alzheimer dementia
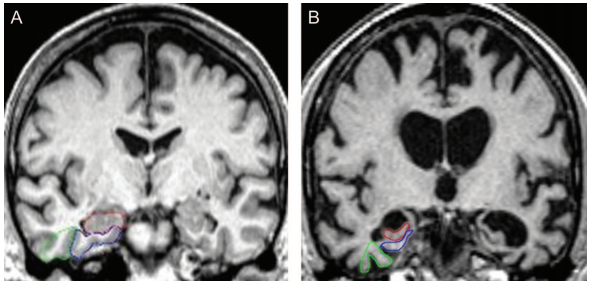
Imaging
Management
- acetylcholinesterase inhibitors (donepezil, galantamine, rivastigmine)
- memantine (moderate-severe stage)
- family support
- treatment of neuropsychiatric symptoms
References
Duara, R. et al. Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology 71, 1986–1992 (2008). https://pubmed.ncbi.nlm.nih.gov/19064880/
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011). https://pubmed.ncbi.nlm.nih.gov/21514250/
Salardini, A. An Overview of Primary Dementias as Clinicopathological Entities. Semin. Neurol. 39, 153–166 (2019). https://pubmed.ncbi.nlm.nih.gov/30925609/